Hermes Ariel Llaín Jiménez
Department of Physical Chemistry
Laboratory of Electrochemical Power Sources
Synthesis and Characterization of Lithium Titanium Oxides as a negative electrode in lithium-ion battery
Hermes Ariel Llaín Jiménez
Supervisor: Dr. hab. Zbigniew Rogulski
Co-supervisor: Dr. Hamankiewicz Bartosz
Lithium Titanium Oxides (LTO) are the materials for anodes (negative electrode) in lithium ion batteries. LTO advantages include operational safety, high capacity at low temperature and excellent high rate performance. The material operates with lack of structural changes during intercalation that helps to avoid cracking into the battery system and allows its use for several charge/discharge cycles increasing the life span of the batteries utilizing LTO1-3.
In order to obtain LTO in a cheaper and easier way, synthesis of the material by means of sol-gel and wet precipitation were carried out. The particle size was determine by Laser Diffraction Spectroscopy indicating an average particle size in between 200 nm and 800 nm. The specific surface area were spotted trough gas absorption-desorption experiments using the BET model. To perform the electrochemical analysis, LTO-carbon-binder electrodes were used as working electrodes within batteries employing metallic lithium as both counter and reference electrodes. After various synthesis experiments optimized condition were find out, displaying an LTO with an specific capacity of at least 160 mAh/g for fresh cells that decreased just approximately 5% after a 25 cycles of a high current analysis (up to 10 C).
The LTO present a high capacity and good behavior in high rate performance, characteristic desired to continue the research of design of new commercial Lithium ion batteries.
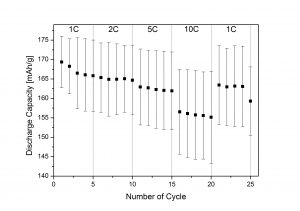 Figure 1. Capacity of LTO-carbon-binder electrodes for the discharge experiments
Figure 1. Capacity of LTO-carbon-binder electrodes for the discharge experiments
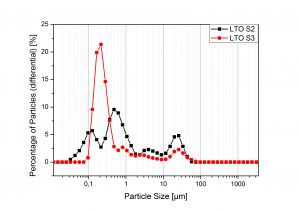 Figure 2. Particle size characterization
Figure 2. Particle size characterization
Literature:
[1] Krajewski, M.; Hamankiewicz, B.; Michalska, M.; Andrzejczuk, M.; Lipinska, L.; Czerwinski, A. Electrochemical Properties of Lithium–Titanium Oxide, Modified with Ag–Cu Particles, as a Negative Electrode for Lithium-Ion Batteries. RSC Adv. 2017, 7 (82), 52151–52164. https://doi.org/10.1039/C7RA10608D.
[2] Krajewski, M.; Michalska, M.; Hamankiewicz, B.; Ziolkowska, D.; Korona, K. P.; Jasinski, J. B.; Kaminska, M.; Lipinska, L.; Czerwinski, A. Li4Ti5O12 Modified with Ag Nanoparticles as an Advanced Anode Material in Lithium-Ion Batteries. Journal of Power Sources 2014, 245, 764–771. https://doi.org/10.1016/j.jpowsour.2013.07.048.
[3] Hamankiewicz, B.; Boczar, M.; Krajewski, M.; Ratynski, M.; Ziolkowska, D.; Czerwiński, A. The Methods of Chemical Modifications of Lithium-Ion Battery Materials. Przeglad Elektrotechniczny 2018, 94, 25–28. https://doi.org/10.15199/48.2018.08.07.
This research was funded by The National Centre of Research and Development through the research grant “Efficient and light photo-rechargeable electric energy storage systems based on solar cell-lithium ion battery or solar cell-supercapacitor structures for special applications”, grant no. TECHMATSTRATEG1/347431/14/NCBR/2018.