Hakim Yusuf
Department of Organic Chemistry and Chemical Technology
Laboratory of Stereocontrolled Organic Synthesis
D-glucosamine-derived amino thiol as ligand for enantioselective addition of organozinc compounds to aldehydes
Yusuf Zaim Hakim
Promotor: prof. dr. hab. Tomasz Bauer
Opiekun: mgr Paulina Morawska
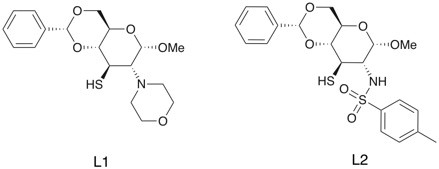
An effort towards the synthesis of two new β-amino thiol ligand, derived from D-glucosamine for the enantioselective organozinc addition has been performed. The first ligand (L1) employs morpholine moiety as the amine protecting group, which was accomplished in the four steps synthesis from amino alcohol of D-glucosamine in 46% overall yield. The thioacetate nucleophilic reactions involving aziridinium ion intermediate was the main key step1. The synthesis of the second ligand (L2) based on sulfonamides functional group is still in the progress. In the preliminary addition reaction of diethylzinc to benzaldehyde, using 10 mol % of L1 at room temperature, enantiomeric excess up to 88% was achieved. The future plan is finishing the synthesis of L2 and catalysis study using variation of catalyst amount, solvent, and temperature.
Literature:
[1] Tseng, S.L., Yang, T.L., Tetrahedron : Asymmetry 16 (2005) 773 – 782.